Nexus Pharmaceuticals Receives FDA Approval for Succinylcholine Chloride Injection USP
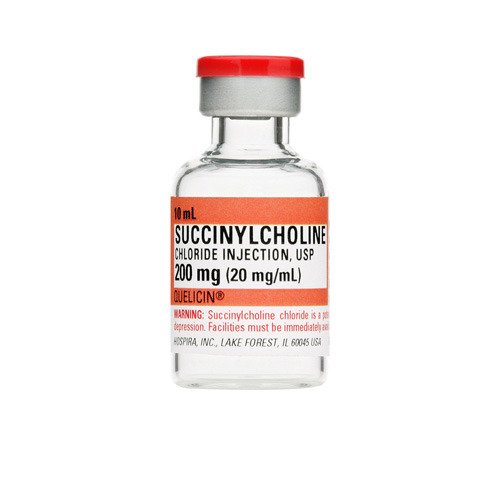
LINCOLNSHIRE, Ill.: Nexus Pharmaceuticals announced it has received U.S. Food and Drug Administration (FDA) approval for Succinylcholine Chloride Injection, USP in 200mg/10mL Multiple-Dose Vials. It is an AP-rated generic to QUELICIN™¥.
“Nexus Pharmaceuticals is very pleased to announce the approval of Succinylcholine Chloride. This critical need drug has seen increased demand due to the COVID-19 pandemic and Nexus will be ready to meet the challenge. This approval further cements Nexus’ commitment to alleviating the drug shortage crisis with sterile injectable products produced exclusively in the US and Europe,” said Mariam S. Darsot, CEO & President of Nexus Pharmaceuticals.
Nexus Pharmaceuticals’ Succinylcholine Chloride Injection will be available in cartons of 25 vials.
Succinylcholine Chloride Injection, USP is a sterile, nonpyrogenic solution to be used as an ultra-short-acting, depolarizing, skeletal muscle relaxant. Succinylcholine is indicated as an adjunct to general anesthesia, to facilitate tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation.















































